March 5th, 2021
EVAPORATION
understanding water / water properties
In this article we’re going to delve deep into the topic of evaporation. Such topic can apparently seem trivial, but don’t be fooled: to be fully understood and used in design, evaporation must be treated as a complex and multifaceted phenomenon. Let’s therefore start from the simplest concepts and then venture into the more complex mechanisms and their applications within the purview of architecture.
When we talk about evaporation we refer to the transition to the vapor phase of particles that are in a liquid phase. It is important to remark that the phenomenon occurs on the surface of the liquid only and can happen at any temperature, differently from what happens for the vaporization caused by boiling (which is a bulk phenomenon and, in order to take place, requires the liquid to reach a temperature of 100 degrees Celsius).
Liquids evaporate to a higher or lower extent depending on the forces that keep their particles together, that is depending on intermolecular forces.
Moreover, particle motion is such that, given a particular temperature, particles have a specific kinetic energy. But this energy is not the same for all particles: it is rather an average value connected to the Maxwell-Boltzmann statistical energy distribution of particles. For this reason, the particles’ kinetic energy is to be compared to an average value and can be approximately defined as higher or lower than the average kinetic energy.
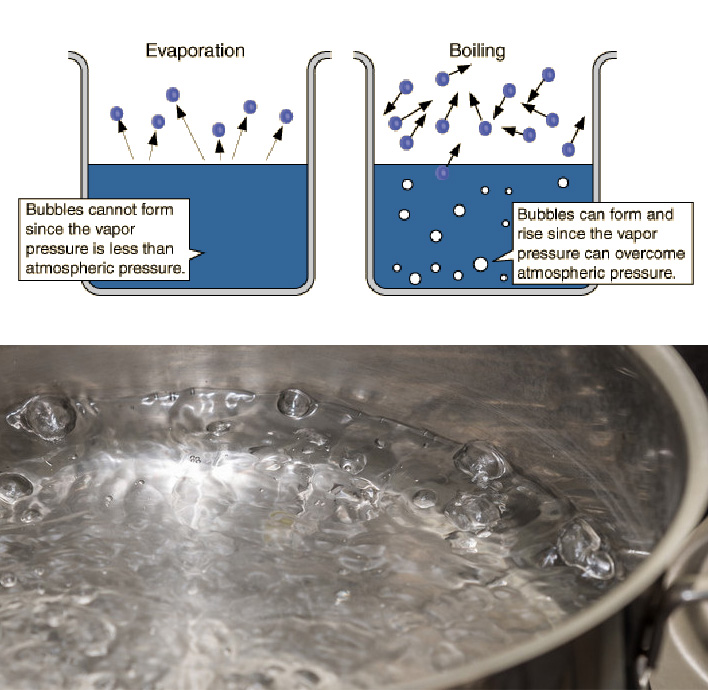
↑ The difference between evaporation and boiling.
Boiling occurs only when the liquid evaporates from its entire volume.

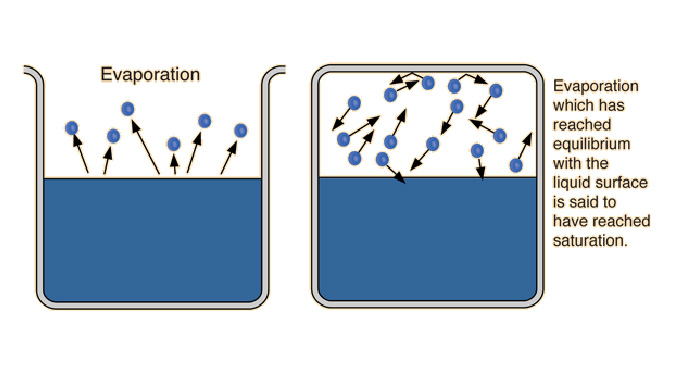
↑ Saturation means balance
This introduction allows us to understand why, in order for a particle to evaporate (that is to drift away from the liquid matter it belongs to), it needs to have energy enough to avoid the attractive action of other particles.
Having ascertained that evaporation can occur at any temperature, the particles with the highest quantity of kinetic energy will be the most eligible to transitioning to the vapor phase.
It also needs remarking that evaporation is a direct function of water temperature and an inverse function of the relative humidity of air (vapor pressure).
It does therefore happen that water absorbs the heat coming from solar irradiance and turns it into the kinetic energy that allows some molecules to overcome the attraction of other water particles and be freed into the air as vapor.
This occurs until the relative humidity of air reaches saturation. When this happens, vapor pressure reaches the state of balance in what is called ‘saturated vapor’, where the vapor particles that return to a liquid phase are as many as the ones the reach a gas phase.
When evaporation happens in a closed system, as temperature rises the evaporation flux also increases and when the environment is saturated balance is reached.
This balance is such that, within a specific time frame, each evaporated molecule will approximately correspond with another going back to the liquid phase.
On the other hand, when evaporation happens within an open system like any puddles or reflecting pools, evaporating particles dispel in the atmosphere and the volume of the liquid gradually decreases until it disappears. If this happens without any heat being brough from the outside we specifically refer to this as spontaneous evaporation.


↑ Examples of closed and open systems
On the basis of what we’ve said so far, we can state that the wind and movement of air are also generally capable of affecting the phenomenon of evaporation: by contributing to containing the increase of relative humidity of air in the surrounding environment, they encourage evaporation.
And indeed, contrary to common belief, air particles blown over the surface of water are capable of speeding up its cooling down. But, as we’ve just seen, this doesn’t happen at all for conduction-related phenomena.
Trivia
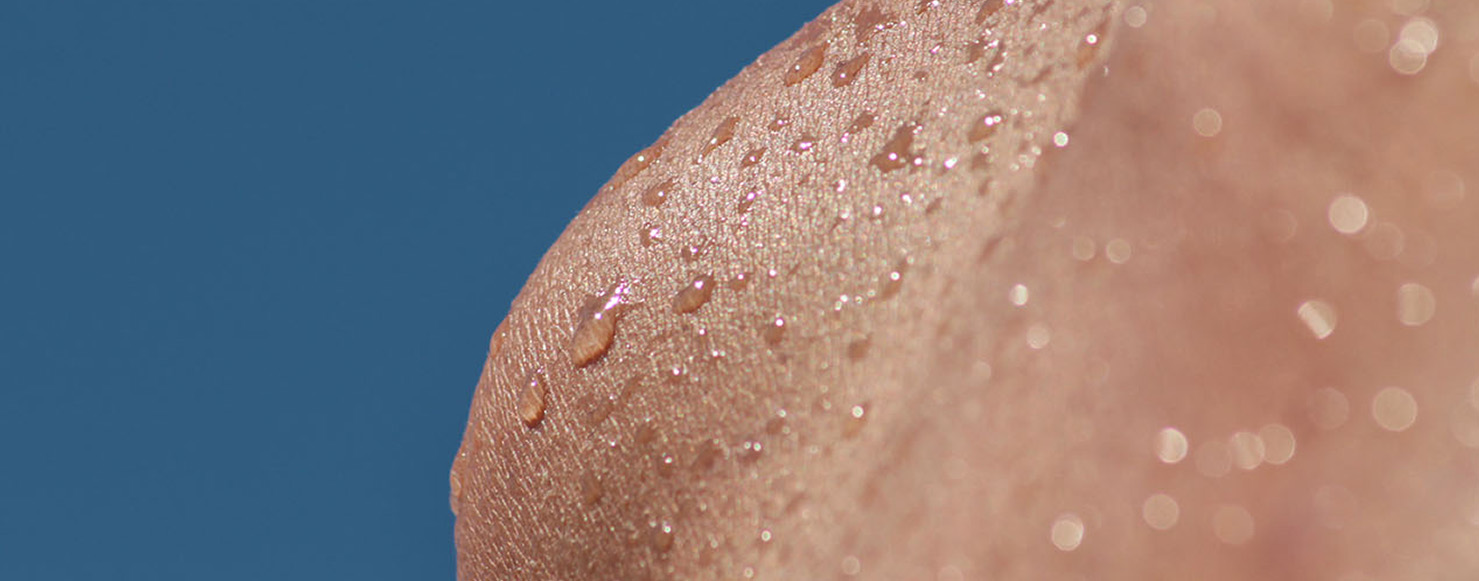
What happens when we get the back of our hands wet and blow air on them immediately after?
We feel a fresh sensation. But is it just a feeling or are we actually blowing fresh air on our hands?
In fact we are blowing 37°C-air on a surface, the one of the back of our hands, whose temperature is normally around 34-35°C. We are therefore blowing hot air on a slightly cooler surface. Why, then, do we feel fresh?
The answer – simple, though not so predictable – is that the secret all lies in the evaporation of that small amount of water that has gotten the back of our hands wet.
The transition of phase of a liquid to the gas phase is indeed the natural phenomenon though which humankind, from ancient times, has managed to create rudimental cooling systems.
Among the many examples we can refer to, let us just think about the aluminum flasks used by our great-grandfathers during World War 1. Specifically, such flasks were based on a simple coating fabric which, once got wet with water, could effectively cool down its own content. And here goes what was happening there from a scientifical point of view: the water evaporation phenomenon outside of the aluminum flask, a prime conductor, managed to subtract heat from the water inside.
Anyway, the evolution of the homeostasis system in the homo sapiens is based on the same principle: when it’s hot, the evaporation of perspiration contributes to the cooling down of the skin surface and, therefore, to maintaining a constant body temperature.

↑ ↑ The soaked padded case of a military water bottle cools the liquid inside.
Concepts To Remember
• Endothermic Process
It is the transformation that accounts for an increase in the system’s enthalpy, thus in a transfer of heat from the environment to the system itself.
• Evaporation of Ponds
It is an endothermic process with the absorption of heat from the pond itself.
• Latent Heat
Due to the subtraction of kinetic energy, the enthalpy of the liquid undergoing evaporation decreases by a specific quantity of the so-called latent heat.
• Evaporation Through Nebulizers
It doesn’t implicate a variation of the system’s energy and is thus defined as ‘adiabatic process’.
It is therefore important not to mistake the concepts of evaporation and nebulization for one another.
Thanks to the concepts we’ve just learned, we’ll be soon able to further understand the cooling mechanisms connected with evaporation and, most of all, how these can help us design water features that are not just beautiful, but also useful and functional. Don’t miss out on the next articles!